Artificial Tears Reca For Eye Strain 2025. Ezricare artificial tears are preservative‐free, multi‐dose, readily accessible eye drops containing carboxymethylcellulose sodium. Artificial tears play a critical role in the management of dry eye disease (ded), providing patient symptomatic relief and improving.
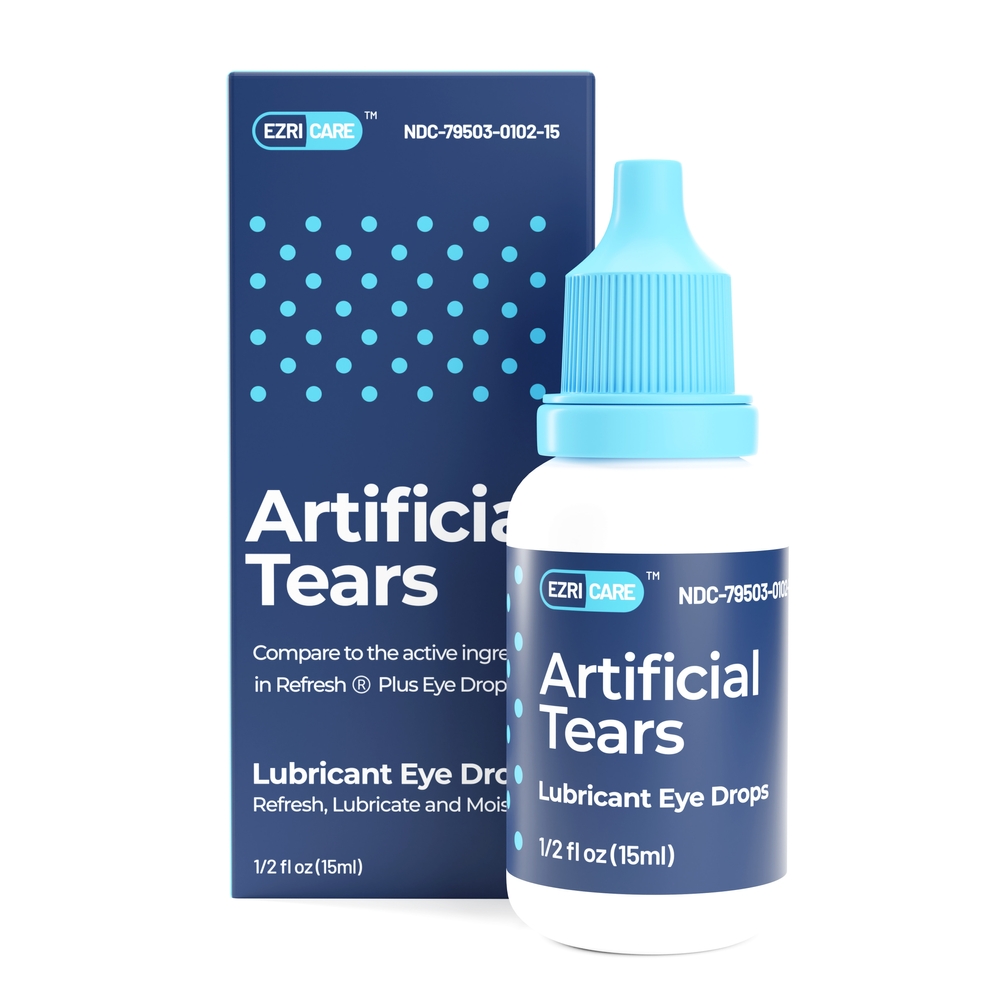
Artificial tears remain the cornerstone for managing dry eye disease. Brs analytical service is voluntarily recalling over 75,000 cases of eye products, including various types of artificial tears, according to a fda enforcement report. An artificial tear solution is one of five ophthalmic products now under recall due to lapses in manufacturing processes, according to the fda.






.jpg)
.jpg)

The Fda And Avkare Have Reported A Recall Of Eye Care Products, Including Drops And Artificial Tears, From Brs Analytical Service.
Artificial tears play a critical role in the management of dry eye disease (ded), providing patient symptomatic relief and improving. Review the facts behind the recent artificial tear recalls and learn about fda protocols and recommendations, with a downloadable list of all recalled drops. Brs analytical service is voluntarily recalling over 75,000 cases of eye products, including various types of artificial tears, according to a fda enforcement report.
An Artificial Tear Solution Is One Of Five Ophthalmic Products Now Under Recall Due To Lapses In Manufacturing Processes, According To The Fda.
Ezricare artificial tears are preservative‐free, multi‐dose, readily accessible eye drops containing carboxymethylcellulose sodium. Artificial tears remain the cornerstone for managing dry eye disease.